Abstract
Background: Belgian ibrutinib Real-world Data (BiRD) is a multicenter, non-interventional, observational, cohort study of adults with a confirmed diagnosis of chronic lymphocytic leukemia, MCL, or Waldenström's macroglobulinemia.
Aim: To assess the effectiveness and safety of ibrutinib treatment in relapsed/refractory MCL in routine clinical practice.
Methods: Eligible patients initiated reimbursed ibrutinib treatment upon or after commercial availability in Belgium (August 1, 2015); or participated in the Medical Need Program and switched to reimbursed ibrutinib with commercial availability. The primary outcome measures were progression-free survival (PFS) and overall response rate (ORR); secondary outcome measures included overall survival (OS), time to next treatment (TTNT), and safety. Data were collected both prospectively (pro) and retrospectively (ret). Ret collected adverse events (AEs) included only those considered related to ibrutinib, whereas pro collected AEs included all treatment-emergent AEs (TEAEs); therefore, TEAEs/AEs are reported separately for the pro/ret groups. Total population effectiveness results were adjusted with left truncation. We report results from the third interim analysis (March 31, 2020 cutoff) for patients with relapsed/refractory MCL, with a median follow-up of 35.8 months.
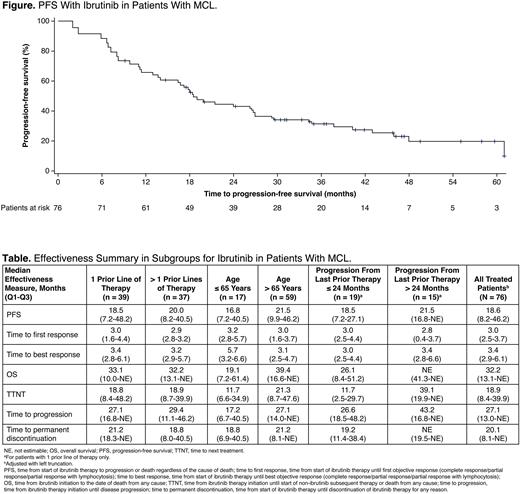
Results: The effectiveness and safety populations included 76 patients (pro, n = 20; ret, n = 56). Median age at ibrutinib initiation was 74 years (range, 47-88); 76.3% were male; median time from diagnosis to ibrutinib initiation was 2.8 years (range, 0.1-17.3). Most patients received 1 (51.3%) or 2 (31.6%) prior lines of therapy, which was most frequently combination therapy (72.4%). Common therapies received in the most recent prior line of treatment were R-CHOP/R-miniCHOP (23.7%), R-DHAP/R-DHAX/R-DHAC (14.5%), alternating R-CHOP/R-DHAP (11.8%), bendamustine (10.5%), and R-bendamustine (10.5%). Complete response (CR; 58.2%) or partial response (PR; 17.9%) was the most common best response to most recent prior therapy. The majority of patients progressed (87.7%) following the most recent prior therapy, and median time from progression to initiation of ibrutinib was 0.72 months (range, 0-25.1). Lymphadenopathy and/or splenomegaly (56.6%) was the most common reason for initiating ibrutinib treatment. Ibrutinib was initiated at 560 mg/day in 85.5% of patients, and 37 patients (48.7%) had ≥ 1 dose modification. With ibrutinib treatment, median PFS was 18.6 months in the overall MCL population (Figure), and 21.5/26.8 months in the pro/ret groups. ORR was 93.4% (CR 39.5%, PR 53.9%). Median TTNT was 18.9 months (34.9/29.1 months in pro/ret) and median OS was 32.2 months (not estimable/43.2 months in pro/ret). Main effectiveness outcomes stratified according to age, prior line of therapy, and progression ≤ 24 and > 24 months from last prior therapy (for patients with 1 prior line of therapy) are listed in the Table. TEAEs/AEs and serious TEAEs/AEs were reported in 100.0/80.4% and 85.0/50.0% in the pro/ret groups, respectively. TEAEs/AEs led to dose reduction, interruption, or withdrawal in 30.0/8.9%, 45.0/30.4%, and 30.0/17.9% of patients in the pro/ret groups, respectively. TEAEs/AEs of interest in the pro/ret groups were major bleeding (10.0/3.6%), infection (55.0/58.9%), hypertension (10.0/3.6%), atrial fibrillation (15.0/12.5%), arthralgia/myalgia (10.0/7.1%), diarrhea (35.0/17.9%), and rash (10.0/8.9%). Ibrutinib was permanently discontinued in 58 patients (76.3%), most commonly due to disease progression (n = 29, 54.7% [5 patients missing reasons for discontinuation]).
Conclusions: Ibrutinib is an effective treatment for patients with relapsed/refractory MCL in a representative real-world population across various patient subgroups; results are consistent with those from clinical trials (Dreyling et al. Hemasphere. 2022;6(5):e712). TEAEs/AEs were consistent with the known safety profile of ibrutinib.
Disclosures
De Beleyr:Janssen: Current Employment. Lahaye:Janssen: Current Employment, Other: Current Employee with restricted stocks. Maes:Janssen: Current Employment. Andre:Takeda, Bristol-Myers Squibb, Karyopharm, Gilead, Incyte: Membership on an entity's Board of Directors or advisory committees; Roche, Bristol-Myers-Squib, Celgene, Gilead, Abbvie: Other: Travel Grants; Roche, Johnson & Johnson, Takeda: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal